Desalination Machine
Uri Lachish, guma science
![]()
Abstract: Operation of a reversible desalination machine is described. The energy of desalination is calculated.
The machine operates in two modes. In one mode it produces pure water from seawater, and in another mode it produces diluted solution from concentrated seawater solution. The system operates reversibly in both modes and consumes the minimal energy determined by thermodynamics. Inevitable irreversible energy losses are not considered here.
Pure water production:
Figure-1 presents the machine and its principle of operation by a three-stage cycle. The system is built of a central cylinder, connected with valves to three tanks. One (sea connected) tank contains seawater with salt concentration of n1 mol/liter. One tank contains concentrated salt solution of n2 mol/liter, and one tank, separated by a semi permeable membrane, contains pure water.
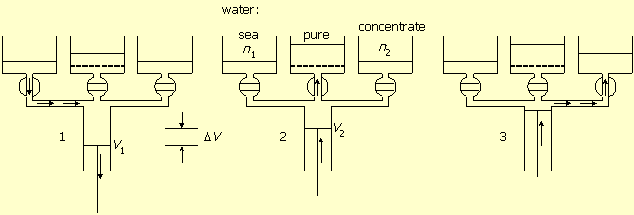
Figure-1: Production of pure water. 1. Seawater pumping in. 2. Pumping pure water out through the membrane. 3. Concentrated solution pumping out.
In stage-1 the seawater valve is open and the other two are closed.
The piston of the central cylinder moves from zero volume to volume V1 and
pumps seawater into it. This movement does not consume energy.
In stage-2 the membrane valve is open and the other two are closed. The piston reverses direction,
pushes pure water out through the membrane, decreases the volume to V2,
and increases the salt concentration to n2.
Applying van't Hoff formula for the osmotic pressure, π = (N/V)RT,
the work of pushing the piston against the pressure will be:
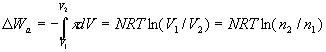 (1)
(1)
where N = n1V1 =
n2V2 is the amount of salt in the cylinder,
R is the gas constant, and T is the absolute temperature.
The machine produces an amount ΔV = V1 -
V2 of pure water in this stage.
In stage-3 the valve to the concentrated solution is open while the other two are closed.
The piston pushes the solution out to the container, returns the cylinder volume to zero,
and completes the cycle.
The work of producing unit volume of pure water is (by equation (1)):
ΔWa/ ΔV = (NRT/ΔV)ln(n2/n1) (2)
The work ΔWa/ΔV increases with the salt concentration n2. If n2 is close to n1, then the logarithm in equation (2) can be approximated by:
ln(n2/n1) ~ (n2 - n1)/n1 (3)
(ln(x) ~ x - 1, x ~ 1). The work per unit volume will then be:
ΔWa/ΔV = n2RT = n1(V1/V2)RT (4)
If n2 ~ n1, then V1 ~ V2 and ΔV << V1, V2. Thus, the system operate in small ΔV strokes and the work approaches n1RT, the energy limit of reversible thermodynamic seawater desalination.
If the system works against higher solution concentration n2, then more energy will be consumed by desalination, according to equation (2). However, the extra energy is not lost, but accumulated in the concentrated salt solution. This energy can be utilized and transformed back to mechanical work by applying the same machine. Figure-2 shows this mode of operation. In this case the cylinder is connected to seawater both directly and via the semi permeable membrane.
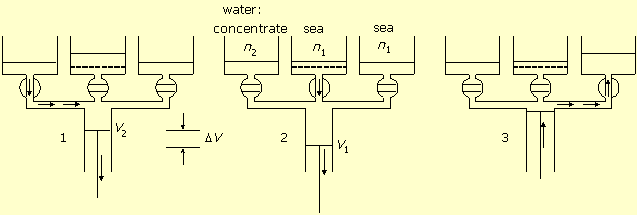
Figure-2: Salt concentration difference transformed to work. 1. Seawater pumping in. 2. Work by volume expansion and water flow in through the membrane. 3. Diluted solution pumping out.
In stage-1 the concentrated solution valve is open. The central cylinder piston moves from zero
volume to volume V2 and pumps in the concentrated solution
n2.
In stage-2 the membrane valve is open. The piston works by expanding the volume from
V2 to V1, while water flows in through the
membrane into the cylinder, and the salt concentration dilutes from
n2 to n1. The work of expansion is:
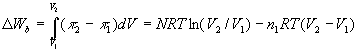 (5)
(5)
π2 = (N/V)RT is the osmotic pressure in the cylinder,
and π1 = n1RT is the osmotic pressure of seawater.
In stage-3 the seawater valve is open and the piston empties the cylinder and reduces its volume to zero.
Two such units can be mechanically coupled. A first unit produces pure water from seawater, and a second unit produces work from difference of salt concentrations. The work output of the second unit is coupled to drive the first unit. The external work, per unit volume of water, that drives the combined system, is given by the difference of equations (2) - (5):
ΔWa/ ΔV = (ΔWa - ΔWb) / ΔV = n1RT (6)
This value, is again the thermodynamic work of seawater desalination.
In summary, desalination is achieved by a single unit, or by two coupled units. A single unit operates in small strokes. In a coupled system one unit produces pure water from seawater, and one unit produces work from the difference of salt concentrations. Both systems can, in principle, arrive at the same thermodynamic efficiency limit.
Production of diluted salt water:
Reversible production of diluted salt water from concentrated salt water is the reverse process of work production from difference of salt concentrations. The process, shown in figure-3, is obtained by reversing both the order of stages and the direction of each operation of figure-2.
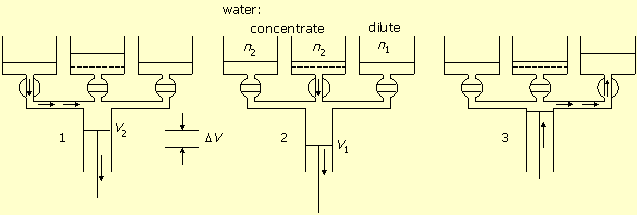
Figure-3: Solution reversible dilution. 1. Concentrated solution pumping in. 2. Dilution by volume expansion and water flow in through the membrane. 3. Diluted solution pumping out.
In stage-1 the concentrated solution valve is open. The piston moves from zero volume to
volume V2 and fills the cylinder with concentrated solution n2.
In stage-2 the membrane valve is open. The piston further moves in the same direction,
water flows in through the membrane into the cylinder, the volume increases from
V2 to V1, and the solution dilutes to
n1. This process, however, can take place only if the osmotic pressure difference,
between the concentrated and diluted solutions, is less than external barometric pressure.
Alternatively, water may be transported through the membrane into the cylinder by applying external
pressure above it. Either way, the energy of the process is given by equation (5).
In stage-3 the diluted solution valve is open, and the piston empties the diluted solution
from the cylinder into the tank.
The energy of seawater dilution (equation (5)) is lower than the energy of pure water production (equation (6)). If stage-1 is omitted, then the system will produce pure water, and the energy in the two equations will be the same.
Conclusion:
The energy of desalination has been calculated for a mechanical system involving a moving piston. However, the result is general. The same energy calculation is valid for any similar thermodynamic reversible desalination process.
on the net: 7th, June, 2000
See:
Energy of Seawater Desalination.
Osmosis Reverse Osmosis and Osmotic Pressure
what they are
By the author:
- "Osmosis Desalination and Carnot", https://urila.tripod.com/Osmosis_Carnot.htm, December 2012.
- "Light Scattering", https://urila.tripod.com/scatter.htm, August (2011).
- "The Sun and the Moon a Riddle in the Sky", https://urila.tripod.com/moon.htm, July (2011).
- "Osmosis and thermodynamics", American Journal of Physics, Vol 75 (11), pp. 997-998, November (2007).
- "van't Hoff's Evidence", https://urila.tripod.com/evidence.htm, October (2007).
- "Osmosis and Thermodynamics", https://urila.tripod.com/osmotic.htm, January (2007).
- "Expansion of an ideal gas", https://urila.tripod.com/expand.htm, December (2002).
- "Optimizing the Efficiency of Reverse Osmosis Seawater Desalination", https://urila.tripod.com/Seawater.htm, May (2002).
- "Boltzmann Transport Equation", https://urila.tripod.com/Boltzmann.htm, May (2002).
- "Energy of Seawater Desalination", https://urila.tripod.com/desalination.htm, April (2000).
- "Avogadro's number atomic and molecular weight", https://urila.tripod.com/mole.htm, April (2000).
- "Vapor Pressure, Boiling and Freezing Temperatures of a Solution", https://urila.tripod.com/colligative.htm, December (1998).
- "Osmosis Reverse Osmosis and Osmotic Pressure what they are", https://urila.tripod.com/index.htm, February (1998).
- "Calculation of linear coefficients in irreversible processes by kinetic arguments", American Journal of Physics, Vol 46 (11), pp. 1163-1164, November (1978).
- "Derivation of some basic properties of ideal gases and solutions from processes of elastic collisions", Journal of Chemical Education, Vol 55 (6), pp. 369-371, June (1978).
Links:
- Thermodynamics Research Laboratory, http://www.uic.edu/~mansoori/Thermodynamics.Educational.Sites_html
- Thermodynamik - Warmelehre, http://www.schulphysik.de/thermodyn.html
- The Blind Men and the Elephant
- My Spin on Lunacy
- Five Weeks in a Balloon
- The first man I saw
- "Faster, Faster!"
- Perfection can't be rushed
- The man higher up
- Brains
- The First-Class Passenger
- other